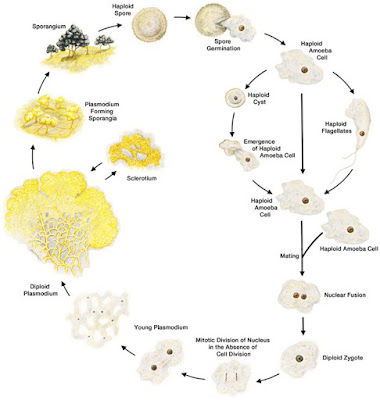
Who is BLOB?
Life cycle
and characteristics
The two
vegetative cell types, amoebae and plasmodia, differ markedly in
morphology, physiology and behavior. Amoebae are microorganisms,
typically haploid, that live primarily in the soil, where they phagocytose bacteria.
In the laboratory amoebae are grown on lawns of live or dead Escherichia
coli on nutrient agar plates, where they can multiply
indefinitely. Axenic culture of amoebae was achieved through
selection of mutants capable of axenic growth. Under conditions of
starvation or desiccation, the amoebae differentiate reversibly into dormant
spores with cell walls. When immersed in water, amoebae differentiate
reversibly into flagellated cells, which involves a major reorganization of the
cytoskeleton.
The
plasmodium is typically diploid and propagates via growth and
nuclear division without cytokinesis, resulting in the macroscopic
multinucleate syncytium. While nutrients are available, the network-shaped
plasmodium can grow to a foot or more in diameter. Like amoebae, the
plasmodium can consume whole microbes, but also readily grows axenically in
liquid cultures, nutrient agar plates and on nutrient-moistened surfaces. When
nutrients are provided uniformly, the nuclei in the plasmodium divide
synchronously, accounting for the interest in using P. polycephalum as
a model organism to study the cell cycle, or more specifically the nuclear
division cycle. When the plasmodium is starved, it has two alternative
developmental pathways. In the
dark, the plasmodium typically differentiates reversibly into a dormant
“sclerotium” (the same term is used for dormant forms of fungal mycelia,
but the myxomycete sclerotium is a very different structure). When
exposed to light, the starving plasmodium differentiates irreversibly into
sporangia that are distinguished from other Physarum species by their
multiple heads (hence polycephalum). Meiosis occurs during spore
development, resulting in haploid dormant spores. Upon exposure to moist
nutrient conditions, the spores develop into amoebae, or, in aqueous
suspension, into flagellates. The
life cycle is completed when haploid amoebae of different mating types fuse to
form a diploid zygote that then develops by growth and nuclear division in the
absence of cytokinesis into the multinucleate plasmodium.
In
laboratory strains carrying a mutation at the matA mating type locus,
the differentiation of P. polycephalum plasmodia can occur without
the fusion of amoebae, resulting in haploid plasmodia that are morphologically
indistinguishable from the more typical diploid form. This enables facile
genetic analysis of plasmodial traits that would otherwise require backcrossing
to achieve homozygosity for analysis of recessive mutations in diploids. Sporangia
from haploid plasmodia generate spores with low fertility, and it is assumed
that viable spores develop from meiosis of rare diploid nuclei in the otherwise
haploid P. polycephalum plasmodia. Apogamic development can
also occur in nature in various species of myxomycetes. In the figure of
the P. polycephalum life cycle, the typical haploid-diploid sexual
cycle is depicted in the outer circuit and the apogamic cycle in the inner
circuit. Note that an apogamic amoeba retains its matA1 mating type
specificity and can still fuse sexually with an amoeba of a different mating
type to form a diploid heterozygous plasmodium—another characteristic that
facilitates genetic analysis.
As the life cycle diagram indicates, amoebae and plasmodia differ markedly in their developmental potential. A remarkable further difference is the mechanism of mitosis. Amoebae exhibit “open mitosis” during which the nuclear membrane breaks down, as is typical of animal cells, before reassembling after telophase. Plasmodia exhibit “closed mitosis” during which the nuclear membrane remains intact. This presumably prevents nuclear fusion from occurring during mitosis in the multinucleate syncytium. In support of this inference, mutant amoebae defective in cytokinesis develop into multinucleate cells, and nuclear fusions during mitosis are common in these mutants.
Cytoplasmic
streaming
The
plasmodium of myxomycetes, and especially that of Physarum polycephalum is
known for its cytoplasmic streaming. The cytoplasm undergoes a shuttle
flow rhythmically flowing back and forth, changing direction typically every
100 seconds. Flows can reach speeds of up to 1mm/s. Within the tubular network
flows arise due to the cross-sectional contractions of the tubes that are
generated by the contraction and relaxation of the membranous outer layer of
the tubes enriched with acto-myosin cortex. In stationary plasmodia
tubular contractions are spatially organized across the entire plasmodium in a
peristaltic wave.
Cytoplasmic streaming is likely to contribute to plasmodium
migration. Here, contraction
patterns are observed to correlate with migration speed. For
dumbbell-shaped microplasmodia, often termed Amoeboid plasmodia, stiffening of
the cortex in the rear versus the front seems instrumental in breaking the
symmetry for the contraction wave to translate into migration.
Cytoplasmic flows enable long-ranged transport and
dispersion of molecules within the cytoplasm. The physical mechanism employed
here is Taylor dispersion. Under
starvation the organism may reorganize its network morphology and thereby
enhance its dispersion capabilities. In fact, the flows are even hijacked
to transport signals throughout the plasmodium network. It is likely that
the feedback of transported signals on tube size underlies Physarum's
capability to find the shortest path through a maze.
Situational
behavior
Physarum
polycephalum has been shown to exhibit characteristics similar to those
seen in single-celled creatures and eusocial insects. For example, a
team of Japanese and Hungarian researchers have shown P. polycephalum can
solve the shortest path problem. When grown in a maze with oatmeal at two
spots, P. polycephalum retracts from everywhere in the maze,
except the shortest route connecting the two food sources.
When
presented with more than two food sources, P. polycephalum apparently
solves a more complicated transportation problem. With more than two
sources, the amoeba also produces efficient networks. In a 2010 paper,
oatflakes were dispersed to represent Tokyo and 36 surrounding
towns. P. polycephalum created a network similar to the existing
train system, and "with comparable efficiency, fault tolerance, and
cost". Similar results have been shown based on road networks in the United
Kingdom and the Iberian peninsula (i.e., Spain and Portugal). P. polycephalum not
only can solve these computational problems but also exhibits some form of
memory. By repeatedly making the test environment of a specimen of P. polycephalum cold
and dry for 60 minute intervals, Hokkaido University biophysicists
discovered that the slime mold appears to anticipate the pattern by reacting to
the conditions when they did not repeat the conditions for the next interval.
Upon repeating the conditions, it would react to expect the 60 minute
intervals, as well as testing with 30 and 90 minute intervals.
P. polycephalum has also been shown to dynamically re-allocate to apparently maintain constant levels of different nutrients simultaneously. In one particular instance, a specimen placed at the center of a Petri dish spatially re-allocated over combinations of food sources that each had different protein-carbohydrate ratios. After 60 hours, the slime mold area over each food source was measured. For each specimen, the results were consistent with the hypothesis that the amoeba would balance total protein and carbohydrate intake to reach particular levels that were invariant to the actual ratios presented to the slime mold.
As the slime mold does not have any nervous system that
could explain these intelligent behaviours, there has been considerable
interdisciplinary interest in understanding the rules that govern its
behaviour. Scientists are trying to model the slime mold using a number of
simple, distributed rules. For
example, P. polycephalum has been modeled as a set of differential
equations inspired by electrical networks. This model can be shown to be
able to compute shortest paths. A very similar model can be shown to
solve the Steiner tree problem. However, these models are externally
consistent but not internally explanatory, and as is usual for modelling they
simplify - in this case assuming conservation of energy. To build
more realistic models, more data about the slime mold's network construction
needs to be gathered. To this
end, researchers are analysing the network structure of lab-grown P. polycephalum.
In a book and several preprints that have not been peer reviewed, it has been claimed that because plasmodia appear to react in a consistent way to stimuli, they are the "ideal substrate for future and emerging bio-computing devices". An outline has been presented showing how it may be possible to precisely point, steer and cleave plasmodium using light and food sources, especially Valerian root. Moreover, it has been reported that plasmodia can be made to form logic gates, enabling the construction of biological computers. In particular, plasmodia placed at entrances to special geometrically shaped mazes would emerge at exits of the maze that were consistent with truth tables for certain primitive logic connectives. However, as these constructions are based on theoretical models of the slime mold, in practice these results do not scale to allow for actual computation. When the primitive logic gates are connected to form more complex functions, the plasmodium ceased to produce results consistent with the expected truth tables.
Even though
complex computations using Physarum as a substrate are currently not
possible, researchers have successfully used the organism's reaction to its
environment in a USB sensor and to control a robot.
Senses
Chemosensation.
Food source chemosensation (taste).
The surfaces of P. polycephalum cells have glycoconjugate receptors for wheat germ agglutinins, Ricinus communis agglutinins, concanavalin A, and soybean agglutinins.
Innate immunity